Molecular sieve technology works on the principle of absorption and diffusion of moisture along with the heat of the system air. Recovery and transfer of moisture not only enhances heat recovery efficiency, but also increases the relative humidity of the supply air. This is particularly desirable when the outdoor air is at a lower relative humidity and would otherwise require the use of energy intensive and problematic mechanical humidifiers.
Systems employing air-cooling to maintain the 19°C (or lower) supply air in summer will also enjoy a reversal of the heat recovery to 'pre-cool' incoming air. That process is extended with the enthalpy recovery equipment to give pre-dehumidification of the incoming air. This will further increase system operating efficiency.
Molecular sieves are effectively thermal wheels whose heat absorbing metal-foil substrate has been coated with a zeolite. This is an aluminosilicate material which can absorb and diffuse water vapour while excluding other particles that can be present in the air.
A close look at the structure of the coated substrate enables one to appreciate the mechanisms that bring about such clean enthalpy transfer. The first is the absorption of the moisture molecule from the air with higher vapour pressure, and subsequent diffusion into lower vapour pressure air. This is performed without need of regeneration, which is necessary when desiccants are used for the purpose.
More significantly, all pathogens and substances other than the water molecule are excluded from the transfer process.
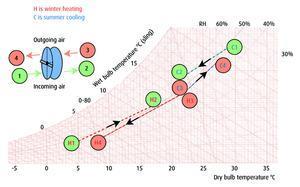
In winter, cold dry air is drawn into the ahu via a filter and passed through the foil substrate-wound enthalpy exchange wheel. The warm humidified air is then delivered to rooms through displacement terminals. The warmed, humid, contaminated air is extracted from the rooms and ducted to the enthalpy exchange wheel. Here, heat and moisture is transferred between outgoing and incoming air streams before being discharged.
Figure 1 shows the relative sizes of some typical airborne contaminants. The illustration shows how the water vapour molecule, at 2.8 angstrom, fits snugly into the made-for-purpose 3 angstrom 'caves' of the particular zeolite. All other materials present in the airstream are excluded. The coating produces an exceptionally slippery surface to the metal foil substrate, far more slippery than that commonly used for non-stick cooking pans.
The technology in practice
Clearly, a zeolite coating to a thermal wheel would bring many benefits to the performance of ventilation systems. Hoare Lea's collaboration with the US firm Healthy Buildings International brought assurances over the efficacy and reliability of the equipment from a large range of users.
Extensive tests conducted by the Georgia State University established both the absence of anything but pure moisture in the transfer. The tests also confirmed the exceptional thermal performance of the Semco molecular sieve enthalpy wheel.
Given these assurances, Hoare Lea considered using the technology in the ventilation systems being designed for the 20 000 m2 headquarters building for Bristol & West plc at Temple Quay in Bristol. Previous Bristol & West buildings had employed steam vapour humidifiers, but for the new building the client was enthusiastic about using the molecular sieve method of enthalpy recovery.
It was chosen over other methods, not only in terms of energy performance but also in consideration of maintaining the highest possible indoor air quality. High indoor air quality is one of the prime features of displacement ventilation, and that should not be compromised by any part of the installation. The particular concern over indoor air quality was to ensure that, while the moisture is being transferred between outgoing and incoming air streams, no contaminant or infection should accompany it.
Design conditions
Within the bands of a comfortable temperature, the relative humidity of the indoor air is ideally kept at 40-50%. For practical and economic reasons, it may be allowed to increase to 60% in summer and to fall to 30% in winter.
At the Bristol & West, examination of the climate data for the site showed an appreciable period in winter when humidification of the displacement supply air would be needed to meet the minimum level for room relative humidity of 30%.
An important question was whether airborne contaminants would abrade the zeolite coating. Investigations in the USA confirmed the toughness of the coatings, and their ability to withstand even light industrial exhaust airstreams. As a result only standard grade air filters are used at Bristol & West to remove larger (perhaps damaging) particles from the airstreams before they pass through the airways of the wheel.
Four air handling plants, which operate in conjunction with static cooling and heating systems, provide the building's ventilation. Tables 1 and 2 show the seasonal peak performances calculated for the enthalpy wheel of each of the air handling plants. These indicate the savings of energy-use compared to a system with no energy recovery facilities.
The annual operating performance has also been estimated using a TAS weather file. A sample of outdoor enthalpy distribution and performance of energy recovery is illustrated on the diagram (figure 2).
It is anticipated that the annual reduction of energy-use achieved by the molecular sieve enthalpy recovery device in the Bristol & West building will amount to 800 000 kWh/y for air heating (70%) and 60 000 kWh/y for air cooling and dehumidification (20%). In turn this should produce an annual reduction of some 233 tonnes of CO2 emissions. These results will be achieved with no compromise to the high standard of air quality specified in order to match the high performance indoor climate demanded for enhanced user satisfaction and productivity.
Continuous monitoring of the temperatures and relative humidity of the incoming and outgoing airstreams are set to start shortly, along with measurements of the prevailing airflow rates. This study should help confirm the hoped-for improvements in energy efficiency and indoor air quality. It will also be useful to know the relationship between the annual amount of energy recovered against the amount of energy used in driving the air through the wheel's airways.
This work will lead to accurate information on energy costs and CO2 emissions, the reliability and maintenance requirements of the equipment, and the system's contribution to indoor air quality.
The future
Purpose-made zeolites can be anticipated to feature in more building services applications. Already they are employed to remove some specific solvents from industrial process and they are also used in the octane manipulation of fuels.
Their wider use on odour control can also be expected, and it would seem certain that ingenious engineers will find many more ways to exploit this 'dog discovered' phenomenon.
Meanwhile, there is every good reason for the use of molecular sieve enthalpy recovery to be seriously considered for all-air systems and especially for displacement ventilation.
The zeolite phenomenon
While zeolites are used widely in process industries, they are not yet a common substance of a building services engineer’s palette. An apocryphal tale, told by Dr Keith Franklin, relates their discovery in 1756 by a Swedish mineralogist, Axel Crondstedt. The story goes that Crondstedt was out for a morning walk with his dog when the hound dug up a rock and wouldn’t give up chewing on it. He named this particular zeolite “stilbite”, for the obvious reason that the dog was still chewing it when he got home. Crondstedt went on to discover a selection of natural minerals which exhibited the strange phenomenon of emitting steam when heated. Hence the other origin for the name Zeolite is from the Greek Zeo, from Zein – to boil, and Lithos – stone. Apart from fake magic, little use was made of zeolites until the 1940’s when Union Carbide produced the first man-made zeolite. Developed in the manner of the catalyst, such zeolites have led to many fit-for-purpose products. One such zeolite is applied as a coating to a heat absorbing substrate of an enthalpy-recovery thermal wheel.Downloads
Figure 1
Other, Size 0 kb
Source
Building Sustainable Design
Postscript
Terry Wyatt FCIBSE is director of research and development at consulting engineer Hoare Lea. Justin Spencer BSc (Hons) is executive engineer at consulting engineer Hoare Lea. For more information about Semco products, visit http://www.semcoinc.com/body_index.html




















No comments yet