This CPD module, sponsored by Aquabion, explores the harmful consequences of limescale build-up in both domestic and industrial hot-water systems, and evaluates the available solutions

How to take this module
UBM’s CPD distance-learning programme is open to anyone seeking to develop their knowledge and skills. Each module also offers members of professional institutions an opportunity to earn between 30 and 90 minutes of credits towards their annual CPD requirement.
This article is accredited by the CPD Certification Service. To earn CPD credits, read the article and then click the link below to complete your details and answer the questions. You will receive your results instantly, and if all the questions are correctly answered, you will be able to download your CPD certificate straight away.
CPD CREDITS: 60 MINUTES
DEADLINE: 24 OCTOBER 2014

INTRODUCTION TO LIMESCALE
Limescale is a hard, chalky substance that builds up in water systems and appliances due to the presence of calcium carbonate deposits in the water supply. These crystallise into blocks of calcite, which settle on the surfaces of the system, causing a number of functional problems and reducing energy efficiency: tests have shown that just 0.06 inches (1.5mm) of limescale can decrease boiler efficiency by 11% (see below).
Limescale is prevalent in “hard water” areas, where the ground contains high levels of calcium and magnesium-containing minerals, such as limestone, chalk and dolomite, and the water supply is pumped from underground (which means that rainwater will have spent time percolating through the soil and rock, dissolving calcium and magnesium). Water hardness is measured by the total concentration of calcium, in milligrams per litre (mg/l). A common definition of hard water is calcium concentration of more than 150mg/l, while soft water has less than 50mg/l.
About 60% of the UK has a geological make-up conducive to hard water, and the water is hardest in central, east and south-east England, including London. In the Lake District and most areas of Scotland, the water is soft because the supply is mainly drawn from surface water, which means that the contact time between water and earth is shorter.
A number of further factors influence the build-up of limescale. The high pH typical of hard water decreases the solubility of calcium carbonate. In addition, high-pressure systems will increase scale formation, as they force more calcium carbonate out of solution.

Why is limescale a problem?
Some £3bn a year is spent on combatting the effects of limescale in the UK alone. It is particularly problematic in systems and devices that heat water, or that heated water passes through, such as boilers, hot water tanks, showers and kettles. This is because calcium carbonate deposits increase rapidly when hard water is exposed to temperatures higher than 60°C. This causes carbon dioxide to be released from the calcium bicarbonate solution in the water, which subsequently solidifies as calcium carbonate. Cold water is also affected, as the inside of any toilet cistern or storage tank will show. Calcification can occur on all types of pipe and tank material.
The build-up of limescale has a number of consequences for hot-water systems:
- It can affect the function of system components, such as pumps, drain valves and spray nozzles. This has an impact on the maintenance and safety performance of the system
- It can reduce the rate of water flow through pipes, which means pipes have to be replaced to maintain flow
- It also acts as an insulator, slowing the transfer of heat from the heating element to the water. This reduces energy efficiency as it will take longer to heat the water
- The sediment can build up on the bottom of heated water tanks, which can overheat the element in electric storage water heaters and the tank material in gas storage water heaters, causing them to fail prematurely.
Limescale build-up does have one advantage, in that it offers some protection against corrosion. This will be significant when we consider the benefits and disadvantages of the available solutions (see below).

EFFECT OF LIMESCALE ON DIFFERENT APPLICATIONS
A number of studies have been conducted into the effect of limescale on the efficiency of hot-water systems and appliances. A 2011 report by the US organisation ASHRAE found that a 0.06-inch (1.5mm) coating of limescale on the heat exchanger in a boiler can reduce its efficiency by an average of 11%. The efficiency decreased in a linear fashion as scale thickness increased.
In 2010, the independent US testing and research facility Battelle Memorial Institute was commissioned by the Water Quality Research Foundation to investigate the effect of limescale on electric storage, gas storage and gas tankless water heaters. The study evaluated the water heaters at 65.5°C for electric storage, 71°C for gas storage and 60°C for gas tankless water heaters, and the tests were conducted using an artificially created water with hardness of approximately 444mg/l of calcium carbonate. The key findings of the study are described below.
Gas hot-water storage heaters
For gas storage water heaters, hard water was found to decrease the efficiency of the equipment from 70.4% to 67.4% over the equivalent of two years. This decrease occurs because limescale and sediment can act as an insulator between the tank burner and the water. The increased insulation also causes heat to build up in the steel tank and glass lining, which can cause the glass to dissolve, degrade the steel and expose the tank shell to corrosion.
The test data was used to establish equations that could predict the performance of heaters over time depending on the hardness of the water. The findings revealed that, over the course of 10 years, every 86mg/l of calcium carbonate deposits leads to a drop in efficiency of 4%, and a corresponding 4% rise in running costs, when using 190l of hot water a day (for means of comparison, a 2008 Energy Savings Trust report found the average UK household hot water consumption to be 122l a day). In very hard water areas, with 512mg/l calcium concentration, this equates to a 24% drop in efficiency.
When using soft water, the heaters maintained the original factory efficiency rating over a 15-year lifetime.

Electric water heaters
The study found that every 86mg/l of calcium carbonate causes 180g of rock-like limescale to build up on the bottom of the tank every year, and that up to 13.6kg can accumulate in these heaters over time. This can bury the heating element, increasing its operating temperature and shortening its lifespan.
Tankless heaters
When using hard water, the efficiency of tankless heaters was found to decrease from 80% to 72% over 1.6 years, after which they failed due to the pressure sensor or other control device being plugged with limescale. After descaling, the efficiency of the tank returned to 77%.
Domestic appliances
The Battelle study also examined the effects of hard water on a number of domestic appliances. Showerheads using hard water were found to lose 75% of the flow rate in less than 18 months. Taps using the same water could not maintain the specified 4.7l/min flow rate because of the collection of limescale on the strainers, which were almost completely plugged after 19 equivalent days of testing.
Dishwashers and washing machines were operated for 30 days and 240 completed wash cycles on soft and hard water sources. The units using soft water were almost completely free from limescale build-up, but the inside of the units using hard water showed the need for descaling.
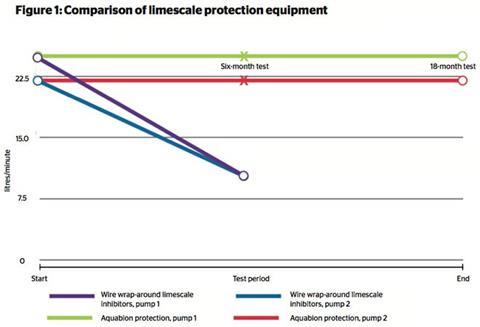
SOLUTIONS TO LIMESCALE BUILD-UP
Products are available to soften hard water, reducing energy costs and preventing component failure. However, it is important to consider the cost and time involved in these processes, as well as potential side-effects. Systems include:
Salt-based water softeners
These are one of the most common approaches to reducing limescale. Using a process known as “ion exchange”, the addition of sodium carbonate allows the water to be softened by exchanging sodium ions, which are soluble, for the insoluble calcium ions. The sodium carbonate is flushed through the system with the water.
One disadvantage of water softeners is that, as salt has to be run through the system on a regular basis, it is not recommended to drink the water. This can become mandatory when the water hardness is above 400mg/l. Weekly maintenance can also be time-consuming, particularly in industrial water systems.
Water conditioning using zinc sacrificial anodes
One alternative to a water softener is a product based on a sacrificial zinc anode. Sacrificial anodes are protection systems commonly used on the hulls of ships and pipelines to prevent corrosion. They are made from a metal with a more active voltage than the structure they are protecting, which means that the anode corrodes in preference to the other metals - hence it is “sacrificial”.
In domestic and industrial water systems, the zinc anode releases zinc ions into the water, which influences the way the calcium carbonate crystallises. The zinc initially reacts with carbon dioxide to form zinc carbonate, or smithsonite, crystals, but as the zinc dissolves it leaves soft needle-like crystals of aragonite, a different form of calcium carbonate to the hard blocks of calcite associated with stubborn limescale. The minerals in the water therefore remain in suspension, rather than sticking onto the surfaces, which means they can be flushed through the system with the water. The anode works both to break down existing limescale encrustations (in certain conditions ) and to prevent further deposits.
The advantages of using zinc anodes are that the water is drinkable and, once installed, they require no further maintenance until the anode needs replacing. Moreover, because the water is not softened, zinc-anode systems offer protection from corrosion as the zinc carbonate also forms a thin protective layer on the surfaces of the system.



How to take this module
UBM’s CPD distance-learning programme is open to anyone seeking to develop their knowledge and skills. Each module also offers members of professional institutions an opportunity to earn between 30 and 90 minutes of credits towards their annual CPD requirement.
This article is accredited by the CPD Certification Service. To earn CPD credits, read the article and then click the link below to complete your details and answer the questions. You will receive your results instantly, and if all the questions are correctly answered, you will be able to download your CPD certificate straight away.
CPD CREDITS: 60 MINUTES
DEADLINE: 24 OCTOBER 2014
Privacy policy
Information you supply to UBM Information Ltd may be used for publication and also to provide you with information about our products or services in the form of direct marketing by email, telephone, fax or post. Information may also be made available to third parties. UBM Information Ltd may send updates about Building CPD and other relevant UBM products and services. By providing your email address you consent to being contacted by email by UBM Information Ltd or other third parties. If at any time you no longer wish to receive anything from UBM Information Ltd or to have your data made available to third parties, contact the Data Protection Coordinator, UBM Information Ltd, FREEPOST LON 15637, Tonbridge, TN9 1BR, Freephone 0800 279 0357 or email ubmidpa@ubm.com. View our full privacy policy at www.building.co.uk/cpd


























No comments yet